How to Control a Fruit Fly with Your Smart Phone
Have you ever wanted to control the behaviour of a fruit fly larvae with your phone? Well, probably not, it is a pretty wild idea – but it has been made possible by researchers at Leipzig University and the Kurt Schwabe Institute for Measurement and Sensor Technology. Why ever would anyone want to do such a thing, you ask? Well, first I must introduce the ground breaking neuroscience technique optogenetics. This technique has helped scientists to understand how our memories are stored and come up with new ideas for how to restore hearing in the deaf, among many other things.12
Information - like the taste of the chocolate you’re eating or the instruction to your arm to swing a badminton racket - travels around in the body as electrochemical signals carried by cells called neurons. Neuroscientists are eager to understand how these neurons work with each other to create complex behaviours, like language, memory, and emotion. It is very difficult to do this by just observing the neurons in action – there are so many different things going on, how do we know which are relevant to our specific research question?
Optogenetics provides neuroscientists a way to label neurons they are interested in and control their electrical signals using light.3 “Opto” refers to light. “Genetics” refers to the fact that genetic manipulation of cells is required for the technique. By being able to directly manipulate the firing of their favourite type of neuron, scientists can ask much more interesting questions about how those neurons work.
So how does optogenetics actually work? The electrochemical signal carried by neurons is actually charged atoms called ions flowing in and out of cells through ion channels. These ion channels are like bouncers at a club. When the club is closed, nobody gets in – so when the ion channel is closed, no ions flow through it. When the club is open, only people approved by the bouncer can enter. For example, only sodium ions are permitted through a sodium ion channel – any other ions are turned away!
In optogenetics, we genetically engineer an animal or group of cells to produce an ion channel that is opened by light in specific types of cell (for example, a subtype of neuron). Depending on the type of ion channel, its opening may increase or decrease the electrical activity of the neuron. Thus, with this elegant design, neuroscientists can perform the experiments of their dreams… Almost.
There are some limitations to optogenetics. It relies on us being able to deliver light to the neurons we want to stimulate. If these are deep in the brain of a mouse, this may be problematic. Moreover, neuroscientists want to be very exact in when and where they stimulate. This means that we need a light source that can turn on and off very quickly, and can emit very small, focussed rays of light.
Researchers have experimented with projectors, lasers, and organic LEDs to try and find the perfect light source. But could it be in your pockets? That’s what Ilenia Meloni and colleagues set out to find out in their recent journal article published in Scientific Reports.4 They used a Huawei Honor 8 smart phone as a light source for an optogenetics experiment. Smart phones have high refresh rates, so should allow very good control over when the light is turned on and off. Moreover, it is possible to write programs that make the smart phone screen display whatever you want! This means scientists can have free rein when designing their experiments.
Fruit fly larvae were genetically engineered to produce light-activated ion channels in their motor neurons – the neurons responsible for muscle contraction. These ion channels are known as inhibitory, because they decrease the electrical signal of neurons. Thus, opening of these ion channels relaxes the muscles of the larvae.
The larvae expressing the inhibitory ion channels were placed on top of a smart phone! When the screen was turned on, the larvae appeared to relax and stretch out. This is because they are no longer able to contract their muscles.
In contrast, the authors expressed excitatory ion channels in the muscle cells of a different group of larvae. When the screen is turned on below the larvae producing the excitatory ion channels, they curl up because all of their muscles contract at once! You can see this in the pair of pictures below.
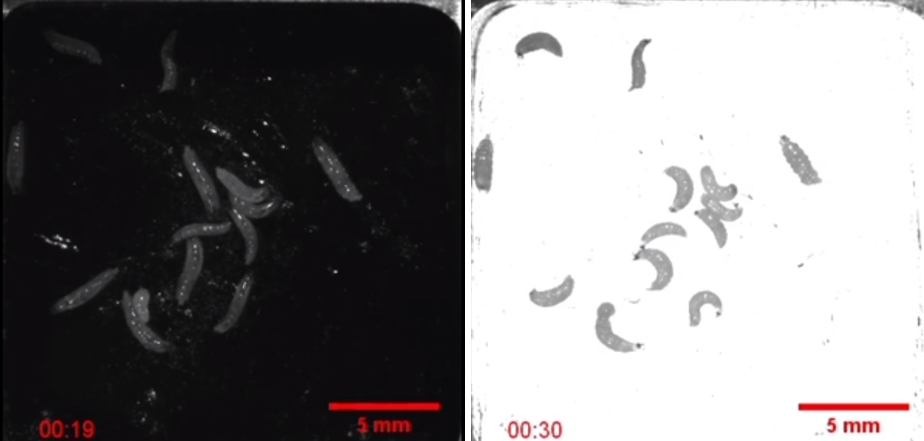
Credit: Meloni et al. 2020. Screenshots of supplementary video S2. Creative Commons Attribution 4.0 International License.
In another experiment the researchers labelled pain neurons with excitatory ion channels. When light hits these neurons, the larvae will feel pain. The experimenters placed these larvae on a screen displaying a donut of light that moves gradually from left to right. Observe in the video below (tweeted by one of the paper’s authors) how the larvae wriggle to stay in the centre of the donut – away from the light.
https://twitter.com/i/status/1321831389709377538
This research paper describes a very simple and easily customisable protocol that other scientists can use to perform optogenetic experiments on not only fruit fly larvae, but potentially also adult fruit flies and other small animals they use in experiments, such as nematodes. Although these animals may seem far removed from humans, they are often used by researchers as models, within which we can study basic biological processes ethically and efficiently. Many important discoveries have been made in these simple organisms.
The authors also point out that optogenetics using smart phones could be a very engaging training exercise for budding neuroscientists – and they tried it out on their own students and their smart phones with much success!
No doubt the weird and wonderful world of optogenetics will continue to be a route to new scientific discovery for many decades to come, and I look forward to what comes next.
References
1. Hernandez VH, Gehrt A, Reuter K, et al. Optogenetic stimulation of the auditory pathway. J Clin Invest. 2014;124(3):1114-1129. doi:10.1172/JCI69050
2. Liu X, Ramirez S, Pang PT, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381-385. doi:10.1038/nature11028
3. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263-1268. doi:10.1038/nn1525
4. Meloni I, Sachidanandan D, Thum AS, Kittel RJ, Murawski C. Controlling the behaviour of Drosophila melanogaster via smartphone optogenetics. Sci Rep. 2020;10(1):17614. doi:10.1038/s41598-020-74448-4